Abstract
Background and Objective: Molecular testing and treatment patterns for patients (pts) diagnosed with acute myelogenous leukemia (AML) have evolved over recent years. Next-generation sequencing (NGS) technologies allow for detection of somatic gene alterations that have prognostic and/or predictive/therapeutic significance. To describe the real-world testing patterns and clinical management of patients with AML, NGS testing, other molecular testing, mutations detected, and targeted treatment administration were analyzed in 2 large community health systems in the US.
Methods: Pts >18 years, diagnosed with AML from January 1, 2015 to December 31, 2020, were identified in the Syapse Learning Health Network, a real-world database with clinical and genomic data from integrated community delivery networks. Study end date was March 31, 2021, allowing for a minimum follow up of 3 months from diagnosis. Pts with less than 2 clinical encounters were excluded. Electronic health records were reviewed retrospectively to analyze molecular biomarker testing patterns, actionable and prognostic biomarkers detected as defined by NCCN guidelines version 3, 2021, and targeted treatments administered. This study received Institutional Review Board (IRB) exemption.
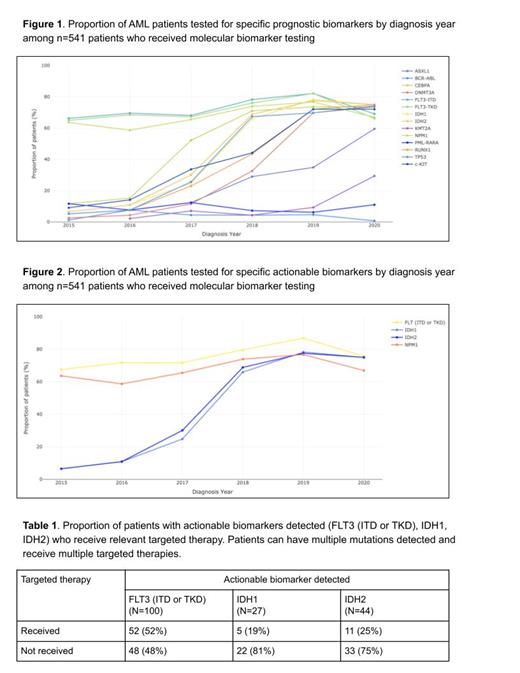
Results: The study included 685 pts with median age at AML diagnosis of 70 and median follow up of 5.4 months. 55% were male, 73% were non-Hispanic white, 10% were non-Hispanic black, 31% had ECOG performance status (PS) 0 or 1 and 16% had ECOG PS > 2 at diagnosis, and 69% had de novo AML. Pts with secondary AML consisted of pts evolving from prior myelodysplasia, myeloproliferative disorder, or aplastic anemia, or therapy related AML. 4% had favorable cytogenetic prognosis, 33% intermediate, and 30% adverse, with the remaining 33% unknown. 541 (79%) pts received either NGS or other molecular biomarker tests. 375 (55%) pts received NGS with or without other molecular biomarker tests and 166 (24%) pts received other molecular biomarker tests only [e.g. Sanger Sequencing, RT-PCR (reverse transcription polymerase chain reaction), PCR]. Pts who did not receive molecular biomarker testing (n=144) were older with median age of 78 and median follow-up of 2 months. There was no statistically significant difference in molecular biomarker testing received between non-Hispanic white and non-Hispanic black population (p=0.275) in the study. There was a statistically significant difference in molecular biomarker testing received between de novo (84% tested) and secondary (67% tested) AML (p=<0.001). NGS tested pts had a median time of 0 days from initial diagnosis to specimen drawn and 13 days from specimen drawn to report generation. 80% of pts first received NGS testing in the upfront diagnostic setting, 15% in the relapse or post diagnostic window (>30 days after diagnosis), with 5% missing relevant dates. 294 (78%) pts had NGS performed on bone marrow aspirate. NGS testing rates rose from 9% of pts diagnosed with AML in 2015, to 77% of pts in 2020. Among pts who received molecular testing (n=541), the proportions of pts tested for specific prognostic and actionable biomarkers by year of diagnosis are found in figures 1 and 2. 204 (38%) pts who received molecular testing had an actionable biomarker detected and of those 204 pts, 70 (34%) received at least one targeted therapy. The proportion of pts with one or more actionable mutations (FLT3, IDH1, IDH2) who receive targeted therapy is presented in table 1 below.
Conclusions: Real-world data provide insights into molecular testing and targeted therapy patterns in routine clinical practice. In this study, testing uptake has increased over time with most pts diagnosed in 2020 receiving testing for FLT3-TKD, FLT3-ITD, IDH1, IDH2 and NPM1. Testing uptake did not differ by race. Among pts with a documented actionable alteration, one third received a targeted therapy. These findings show progress in testing for pts with targetable biomarkers in AML in the community setting, although further increases in testing and faster results could provide additional clinical benefit. Future directions for this work include analyzing patient outcomes.
Coutinho: Syapse: Ended employment in the past 24 months. Berry: Syapse: Current Employment, Current holder of stock options in a privately-held company. Thompson: Doximity: Current equity holder in publicly-traded company; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS (Celgene): Membership on an entity's Board of Directors or advisory committees, Research Funding; Syapse Precision Medicine Council: Membership on an entity's Board of Directors or advisory committees; Lilly: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Elsevier ClincalPath (VIA Oncology): Membership on an entity's Board of Directors or advisory committees; Strata Oncology Advisory Board: Membership on an entity's Board of Directors or advisory committees, Research Funding; UpToDate - Plasma Cell Dyscrasia: Patents & Royalties; TG Theerapeutics: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; LynxBio: Research Funding; Amgen: Research Funding; Epizyme: Honoraria, Membership on an entity's Board of Directors or advisory committees; Denovo: Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Research Funding; Hoosier Research Network: Research Funding. McCracken: Syapse: Current Employment, Current holder of stock options in a privately-held company. Geverd: Syapse: Current Employment, Current holder of stock options in a privately-held company. Law: Syapse: Current Employment, Current holder of stock options in a privately-held company. Wolf: Syapse: Current Employment, Current holder of stock options in a privately-held company. Brown: Syapse: Current Employment, Current holder of stock options in a privately-held company; GenomiCare Biotechnology: Consultancy, Current holder of individual stocks in a privately-held company; Sygnomics: Current holder of individual stocks in a privately-held company. Kuriakose: Alexion: Speakers Bureau; Celgene, Takeda, Pfizer, Kedrion, Apellis, Sanofi-Genzyme, Chiesi, TG Therapeutics, CSL Behring, CTI Biopharma: Membership on an entity's Board of Directors or advisory committees; Via industry sponsored institutional clinical trials.: Research Funding.